Endemic Disease Surveillance Requires Quality Testing and Hospitalization Data
With testing and case data rapidly depreciating in quality, experts are relying more on hospitalization data as a warning sign for COVID-19 outbreaks. A reliable disease surveillance system in the future will need both hospitalization data and testing data to function.
The onset of the COVID-19 pandemic caught almost everyone by surprise. The fact that public health data collection and reporting systems needed to be built from scratch hamstrung public health responses and likely contributed to faster disease spread and preventable deaths. No one wants to experience that again, which means that ongoing public health surveillance will be a necessary component of a post-pandemic world. We need to invest in data systems that can function just like a home security system, warning us when there is a potential danger and giving us time to react, prepare, and alert the proper authorities.
Disease surveillance is not a new idea. Medical and public health experts have been keeping tabs on infectious diseases long before COVID-19 became the most discussed one. Epidemiological monitoring, testing, and case data can serve as an early warning system for hospitals. Instead of reacting to a crisis when emotions are high and while emergency rooms are overwhelmed, hospitals could prepare by shifting resources, obtaining personal protective equipment and instrumentation, and hiring or transferring staff when they are alerted that a new threat is on the way. Surveillance also has many purposes apart from recording disease spread. Samples from disease surveillance labs are analyzed to better understand virus biology, sequence variants, and identify new viruses.
Throughout the COVID-19 pandemic, hospitals and public health officials have relied on accurate, real-time testing and case data to inform their operations. Unfortunately, those data streams are becoming less reliable by the day. The quantity of data that we receive is significantly decreasing due to the widespread use of at-home testing that is rarely reported, reduced institutional testing requirements,1 and fewer people pursuing testing due to how mild the Omicron variant has been compared to Delta.2 Even when COVID-19 transitions to an endemic disease, recording cases and collecting test samples remains critical. These data help researchers to assess vaccine efficacy and design new treatments, hospitals to prepare for surges, epidemiologists to understand disease transmission, and molecular biologists to search for new variants and diseases. Abandoning testing when the pandemic ends would remove an essential component of surveillance.
When testing and case data fail, we then have to rely on hospital admissions data to reflect the current state of disease spread. Thankfully, hospitalization data is some of the highest quality data available, for now. The Centers for Medicare and Medicaid Services (CMS) mandated standardized, robust data collection and reporting from all hospitals as a condition of funding back in Fall 2020.3 The mandate was tied to the declaration of a Public Health Emergency pursuant to the Public Health Service Act by then Secretary of Health and Human Services Alex Azar. The state of emergency has been renewed every 90 days since then, with many major healthcare organizations pleading for current Secretary Xavier Becerra to extend it once again this month.4
While the state of emergency is in effect, the CMS reporting mandates will also likely continue, but what happens once the state of emergency is lifted? Public accessibility and real-time reporting of hospitalization data are still very important for endemic diseases. As we discussed with Dr. Kimia Ghobadi, public availability of hospitalization data enables hospitals to triage more rapidly when overwhelmed and supports informed decision-making about resource and staffing allocations. The CMS data mandates may have been difficult for hospitals to initially enact,5 but now that they are in place, this data reporting should continue regardless of the state of emergency and penalties for non-compliance. However, hospitalization data is not enough.
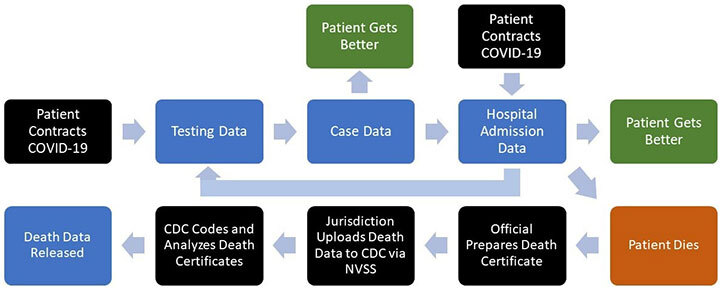
In the flow of COVID-19 data (above), testing and case data are upstream of hospitalization data, encompass all confirmed cases of COVID-19 regardless of severity, and are available much earlier. There is significant value in maintaining testing and case data as part of an early warning system for COVID-19 outbreaks or surges. However, with the continued decrease in testing use by non-hospitalized individuals,2 obtaining reliable testing and case data for surveillance will require designing dedicated systems.
Random sampling of the population is a promising option for continued disease surveillance.6 Testing of a random set of individuals across the country should give a clear understanding of the current state of COVID-19. One option could be testing samples from random patients who happen to be in clinics or hospitals for other reasons. There would be some privacy concerns, but no more than with current testing, and individuals would have to consent to participate. We could also rely on concerned citizens who want to contribute to public health efforts and donate samples or offer them for a small monetary reward as many research studies are currently performed. That, however, could produce significant sampling bias as the people most likely to help with continued prevention of COVID-19 would be those who are particularly cautious about disease transmission.
Whatever the design, we do need an ongoing system of COVID-19 testing surveillance, which will also feed sequencing datasets that monitor for new variants. A system of random sampling would vastly improve our current sequencing infrastructure, which does not reflect the majority of the country. Instead, most samples come from New York City and Los Angeles.7 This process will also require significant, continued funds, when Congress already appears hesitant to continue funding COVID-19 efforts.8
We don’t want to be surprised again, whether it’s a COVID-19 surge, a new variant, or even a new virus. We built suitable data infrastructure for disease surveillance during this pandemic, but we have to maintain it, adapt it to this new purpose, and provide adequate samples to represent the full geographic and demographic diversity of the United States. No one wants to face something like this again as unprepared as we were. But by not instituting a disease surveillance data system complete with testing data, we are starting back at zero. If history inevitably repeats itself, we should at least be prepared next time.
References
- A. Patil, U.S. colleges that once championed surveillance virus testing are backing away, The New York Times, 04 April 2022.
- M. Maddipatla, M. Roy, Lower testing rates likely reason for falling COVID-19 case reports - WHO, Reuters, 16 February 2022.
- CMS releases guidance on COVID-19 data reporting as a condition of hospitals’ Medicare participation, American Hospital Association, 6 October 2020.
- Association of American Medical Colleges, AAMC, Hospital Groups Urge HHS Secretary to Renew Public Health Emergency, 01 April 2022. https://www.aamc.org/advocacy-policy/washington-highlights/aamc-hospital-groups-urge-hhs-secretary-renew-public-health-emergency. (Accessed 05 April 2022).
- R. Pollack, RE: Interpretive Guidance For CMS-3401-IFC, American Hospital Association, 04 September 2020.
- A. Foddai, J. Lubroth, J. Ellis-Iversen, Base protocol for real time active random surveillance of coronavirus disease (COVID-19) - Adapting veterinary methodology to public health, One Health 9 (2020) 100129.
- Council on Foreign Relations, Data's Role in Preventing the Next Pandemic, YouTube, 16 February 2022.
- E. Cochrane, A.E. Petri, A.J. Khan, Covid News: Senators Announce Smaller Aid Proposal Without Global Vaccine Funds, The New York Times, 04 April 2022.
